![]() Our mission is to decipher the pathophysiological mechanisms of magnesium disorders. By identification of genes involved in hereditary hypomagnesemia, we aim to increase our understanding of the molecular mechanisms of magnesium reabsorption in the kidney. Embedded in the department of Medical BioSceinces of Radboudumc, we study the regulation of magnesium handling by the kidney, as well as, the role of magnesium in disease including diabetes mellitus type 2 and chronic kidney disease.
Our mission is to decipher the pathophysiological mechanisms of magnesium disorders. By identification of genes involved in hereditary hypomagnesemia, we aim to increase our understanding of the molecular mechanisms of magnesium reabsorption in the kidney. Embedded in the department of Medical BioSceinces of Radboudumc, we study the regulation of magnesium handling by the kidney, as well as, the role of magnesium in disease including diabetes mellitus type 2 and chronic kidney disease.
Vision
In our view, the development of next-generation diagnostics and innovative functional methods to measure Mg2+ transport are essential to diagnose and to understand Mg2+ wasting tubulopathies. We envision that patient-specific models are the future of pathophysiological research. Personalized drug screening to identify therapeutics that fit the needs of individual patients will become the new standard for treatment of rare diseases.
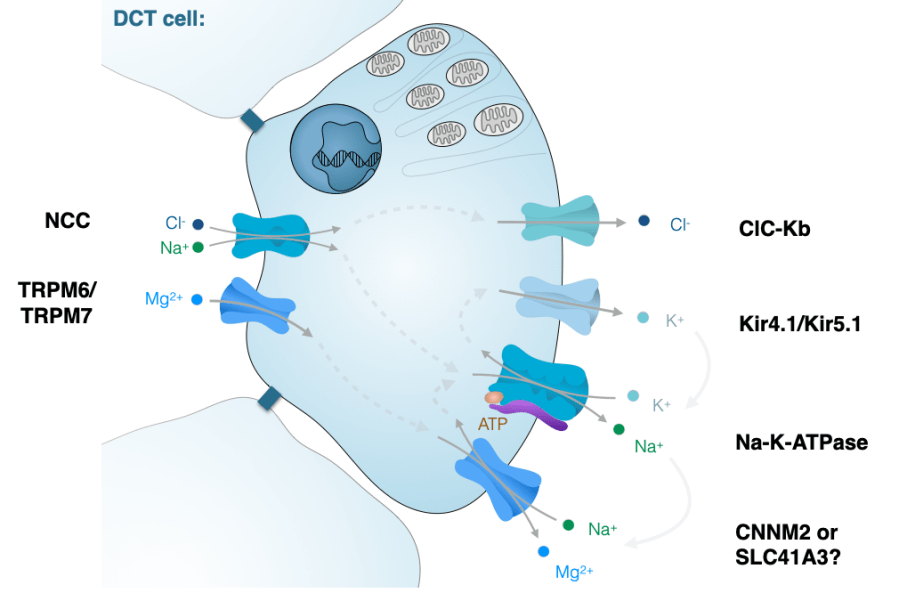
Hereditary hypomagnesemia
Our work resulted in the identification of novel magnesium-wasting tubulopathies caused by mutations in CNNM2, PCBD1, KCNJ16, RRAGD and TRPM7. By linking genetic screening to functional studies, we have provided insights into the mechanisms of magnesium reabsorption in the kidney.
- Mutations in MT-TI and MT-TF cause a Gitelman-like syndrome of hypomagnesemia, hypokalemia and metabolic alkalosis (J Am Soc Nephrol 2022). We demonstrated that energy metabolism is coupled to Na+ reabsorption in the distal convoluted tubule.
- We identified RRAGD mutations in a large multicentric patient cohort with a novel inherited salt-losing tubulopathy, hypomagnesemia and dilated cardiomyopathy. Our findings demonstrate that an activation of mTOR signaling causes this novel disease phenotype, suggesting a critical role of Rag GTPase D for renal electrolyte handling and cardiac function. (J Am Soc Nephrol 2021)
- KCNJ16 (Kir5.1) mutations were demonstrated to cause a novel hypokalemic tubulopathy.(J Am Soc Nephrol 2021) Moreover, HNF1B was shown to be the transcriptional regulator of the Kir5.1 K+ channel, explains why HNF1B patients suffer from hypomagnesemia and hypokalemia mimicking Gitelman syndrome. (Kidney Int 2017).
- CNNM2 mutations were identified in patients with hypomagnesemia, intellectual disability and seizures. We showed in cell models, mice and zebrafish that CNNM2 is essential for the magnesium balance and brain development (PLOS Genetics 2014, Human Mutation 2021, Scientific Reports 2021).
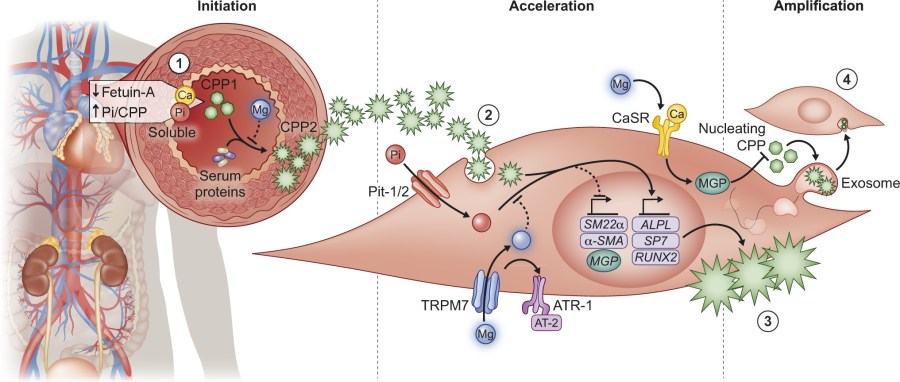
Mineral metabolism in CKD
Our group has shown that magnesium prevents vascular calcification in vitro and in vivo. In bovine and human vascular smooth muscle cells (VSMC), Mg2+ effectively prevented high Pi-induced mineralization, as well as in mouse models of CKD. My team is currently working together with colleagues form the Amsterdam UMC and UMCG in the NIGRAM2+ consortium to translate our findings towards patients.
- In Klotho knock-out mice, high Mg2+ intake prevented aortic calcification and molecular pathways involved in ECM remodeling and inflammation. (Kidney Int, 2020)
- Magnesium was shown to prevent vascular calcification in vascular smooth muscle cell cultures. Our findings indicate that magnesium inhibits crystal formation and thereby prevents calcification. (Sci reports, 2018, Nephrol Dial Transplant 2020).
- Literature research indicates that many studies support an association between serum magnesium and cardiovascular disease risk. A substantial body of in vitro and in vivo studies has identified a protective role for magnesium in vascular calcification. (Arterioscler Thromb Vasc Biol., 2017).
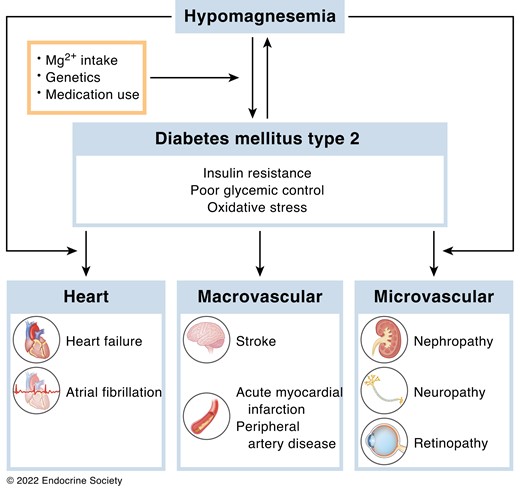
Hypomagnesemia in type 2 diabetes
As 30% of all type 2 diabetics suffers from hypomagnesemia, we aim to effects of magnesium on lipid and glucose metabolism. Our work demonstrates that magnesium affects insulin sensitivity and lipid metabolism.
- Magnesium deficiency abrogates HFD-induced obesity in mice through enhanced eWAT lipolysis and BAT activity. (Diabetologia, 2018). Moreover, low Mg levels contribute to insulin resistance in adipocytes by reducing Akt phosphorylation (Frontiers in Endocrinology, 2022).
- Free fatty acid levels directly reduce the blood magnesium concentration, in part explaining the high prevalence of hypomagnesaemia in metabolic disorders. (Diabetelogia, 2019).
- We determined that 30% of patients with diabetes mellitus type 2 has hypomagnesemia. Blood glucose and triglyceride levels were associated with blood magnesium concentration in a cohort of 400 patients. (Eur J Endocrinol Metab., 2017).